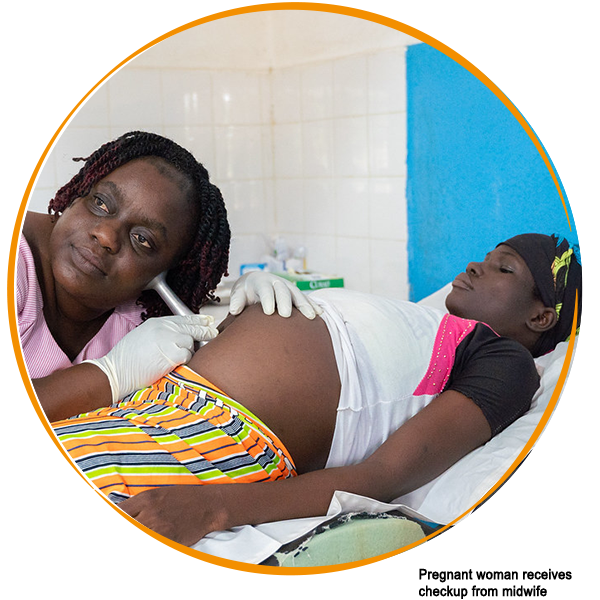
Malaria adapts – so must we
Letter from the Chair and CEO

Mr Alan Court
Chairman of the Board

Dr Martin Fitchet
Chief Executive Officer
In 2024, Medicines for Malaria Venture marked 25 years of commitment to beating one of humanity’s oldest and deadliest foes. Since 1999, working with our partners, MMV has brought forward 17 new medicines, which have effectively treated 711 million patients — a testament to the power of partnerships.
To accelerate progress, last year, MMV launched a bold new strategy charting a course toward a malaria-free future—one rooted in innovation, equity and shared purpose. It reaffirms our mission to develop next-generation tools and ensure they reach those who need them most.
This new chapter comes at a critical time. The World Health Organization’s (WHO) 2024 World malaria report revealed nearly 600,000 malaria deaths in 2023, almost unchanged from 2022. This is unacceptable. Now, more than ever, we must stand together. Let us not allow a preventable and treatable disease to continue taking lives and futures. Let us act with urgency, with compassion, and with the conviction that a world free of malaria is not only possible—it is within our grasp.
Dr Jean Kaseya
Director General
Africa Centres for Disease Control and Prevention
It is time for global health and malaria stakeholders to redouble our commitment to ending this disease. We must urgently prioritize R&D for next-generation antimalarials with high barriers to resistance that can accelerate progress towards elimination. We must also continue scaling up access to existing medicines by improving supply chains and investing more in African countries’ capacity to manufacture their own quality-assured medicines. Now is the time to step up, not shy away from political and financial commitments.
Achievements 2024



Impact in action
In 2024, MMV achieved significant milestones:
- 109 million people in endemic regions received an MMV-supported product
- 36 million patients were appropriately treated for malaria, including:
- 4 million with artemether-lumefantrine dispersible
- Over 1 million with Pyramax® (pyronaridine-artesunate)
- 6 million severe malaria patients treated with injectable artesunate
- 5 million with rectal artesunate
- Seasonal malaria chemoprevention protected 54 million children in 19 countries.
Since 1999, MMV-supported products have effectively treated an estimated 711 million people and 1.3 billion courses of malaria preventive medicine have been received by children.


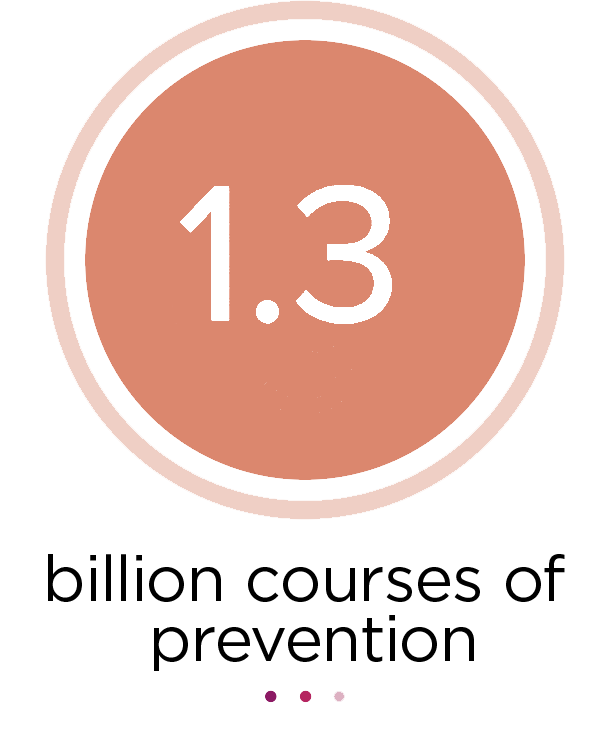
Celebrating 25 years of impact

Throughout 2024, MMV commemorated 25 years of working towards a malaria-free world with our partners. We actively participated in global health events of influence, contributing to discussions on the best strategies to achieve elimination.
Our response

Transforming the antimalarial landscape
In response to the increasing threat of antimalarial drug resistance, MMV and partners are exploring strategies to extend the useful lives of existing antimalarials and to expand their use while they remain effective. We are also co-developing innovative next-generation medicines for prevention and treatment — including novel non-artemisinin alternatives — and making them accessible to the people who need them.

Innovative approaches to malaria prevention
Effective malaria prevention requires deployment of multiple, complementary interventions, including vaccines, medicines (chemoprevention) and vector-targeted tools. Different tools are best suited to different settings and demographic groups, and none provides full protection when used alone. In particular, current malaria vaccines are being used to protect children in some 17 countries. They complement rather than replace chemoprevention and vector-targeted tools: the vaccines protect only against P. falciparum, and protection is partial, decreasing over time.

Eliminating malaria through access and innovation
In addition to preventing and treating clinical illness, elimination involves reducing transmission of parasites between people and mosquitoes. One way of doing this is to use medicines that target different parasite life cycle stages, and several medicines in MMV’s pipeline have shown such transmission-blocking potential. Elimination also requires wiping out all reservoirs of the parasite — including those in asymptomatic carriers, which calls for medicines that can be used in large proportions of the population and present high barriers to parasite resistance.

Expanding impact: from malaria to global health innovation
Many of the systems, tools and processes that MMV has developed can be adapted to support discovery, development and delivery of critical medicines for other diseases, particularly those affecting low- and middle-income countries. We continue to lead in exploring applications of artificial intelligence and machine learning to optimize and accelerate delivery of new products, starting with drug discovery. We are also committed to open engagement with the R&D community, sharing what we learn in our work to defeat malaria.
Research
Translational
Product
development
Access
Research
Translational
Product development
Access
Antimalarial pipeline
Our antimalarial portfolio is the largest ever assembled and comprises 13 compounds in clinical development and three in regulatory review targeting unmet medical needs, including medicines for children, pregnant women and people suffering from drug-resistant malaria. MMV is committed to harnessing machine learning to impact product delivery and quality, ultimately shortening the time it takes to identify compounds that meet demanding target product profiles.
Project of the Year 2024
The promise of protection for a full malaria season
MMV’s independent Expert Scientific Advisory Committee has awarded Project of the Year 2024 to Dr Benoît Bestgen and Dr Paul Willis, representing the R&D long-acting injectables (LAI) team.
In MMV’s first LAI project, MMV371+MMV055, the team did trailblazing work in defining the LAI Early Development strategy and assembling the strong data set that enabled MMV371 to enter Phase 1 in September 2024. The team’s achievements include developing an intramuscular MMV371 formulation potentially appropriate for once-per-season chemoprevention, generating the preclinical safety data needed to advance MMV371 to studies in humans, designing and obtaining regulatory approval for the first Phase 1 study of MMV371 and seeking approval from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) for advancing MMV055 to Phase 1.
Financials
Medicines for Malaria Venture receives sustained funding and support from government agencies, private foundations, international organizations, corporations, corporate foundations and private individuals.
These funds are used to finance MMV’s portfolio of R&D projects as well as specific, targeted access and delivery interventions aimed at making it easier for underserved populations to gain access to life-saving medicines.
Donors
MMV is grateful for the support in 2024 from individual donors as well as the following institutional donors:


Credits
Editors: Doreen Yomoah and Elizabeth Poll, MMV; Annette Dubois, Luminaria Science Writing
Design: Pierre Chassany, ComStone, Geneva, Switzerland
Photographs: Cover: Toby Madden/Transaid; Achievements (clockwise from top left): Elizabeth Poll/MMV, bogubogu/Shutterstock, Omotayo Tajudeen/Swipha/MMV; Impact in action: Greg Lomas/MMV, Daniel San Martin, Mwangi Kirubi/PMI Impact Malaria, Elizabeth Poll, PATH/MMV/PAVE; Our response: Toby Madden/Transaid, Omotayo Tajudeen/Swipha/MMV, Thiago Poncio/MedAccess/MM/PATH, AMISOM Public Information; Project of the Year: Wuxi